Fmoc Based Peptide
Synthesis
Peptide Machines is dedicated
to producing instrumentation that allows our customers optimal and efficient
synthesis of peptides. Our automated solid-phase synthesis instruments enable
the synthesis of custom peptides, peptide libraries, upscaled synthesis, and
many others.
A short historical view.
The peptide bond was
described and accepted as the link between amino acids by 1902. However,
scientists disputed the structural nature of proteins until the end of World
War II after 1951, when Linus Pauling established the models for the
α-helix, and Fred Sanger first sequenced the insulin chains.
[1836 to 1930] The German
physician and physiologist Theodor Schwann isolated a stomach content that
could digest meat in 1836 and called it pepsin, and in 1930, the American
biochemist John H. Northrop purified and crystalized pepsin.
[1946] In 1946, John H.
Northrop won the Nobel Prize in Chemistry with James B. Sumner and Wendell
Meredith Stanley for the isolation, crystallization, and study of enzymes,
proteins, and viruses.
Pepsin breaks down the
protein albumin from egg whites into peptones. Peptones are soluble proteins
formed during the early protein breakdown or digestion stages. Peptone
solutions enable the growth of bacteria in a liquid medium.
The digestion of proteins by
stomach enzymes produces more readily soluble products that can no longer be
coagulated by heat, later named peptides.
In the 19th century, organic
chemists discovered amino acids, first as a family of related molecules and
later as components released from proteins by acid hydrolysis. The amino acid
glycine was identified by 1850 as amino-acetic acid and alanine as
amino-propionic acid. Many amino acids were known for some time before being
recognized as part of the acid hydrolysates of proteins. It took until the 20th
century to discover all 20 amino acids common in proteins. Feeding studies
performed in animals and humans by William C. Rose, an American biochemist,
around 1935 showed that a balanced protein synthesis requires all 20 amino
acids. Rose found that humans and rats must get eight (8) essential amino acids
from food to maintain metabolic balance. These essential amino acids are
leucine (Leu, L), isoleucine (Ile, I), valine (Val, V), threonine (Thr, T), methionine (Met, M), phenylalanine (Phe, F),
tryptophan (Trp, W), and lysine (Lys, L).
[1954] In 1954, Crick and
Watson proposed a list of 20 common amino acids that are actually found in
proteins. The amino acid hydroxyproline found in collagen was considered a
likely modification after protein synthesis.
The German chemist Hermann
Emil Louis Fischer, who received a Nobel Prize in chemistry in 1902 for his
work in sugars and purines, by 1901 achieved the first synthesis of the peptide
linkage, glycyl-glycine. In 1902, Fischer used the term peptide when describing
the bond in a lecture. Fischer studied the problem of specificity in protein
linkage by synthesizing peptides of known compositions and comparing them to
the chemical behavior of the synthetic peptides with the digestion products of
meat. Enzymes in stomach and pancreas extracts could digest the synthetic
peptides made by Fischerís research group. This digestion result showed that
those peptides were like those existing in actual proteins (Fischer
1907).
[1955] The American
biochemist Vincent du Vigneaud received the Nobel Prize in Chemistry for his work on biochemically important sulfur compounds,
especially for the first synthesis of a polypeptide hormone, the peptide hormone oxytocin. Vincent
du Vigneaud first determined the sequence of oxytocin
and chemically synthesized the nonapeptide hormone.
[1960 to 1970] By this time, the
exact sequence of many proteins was known. In 1959, to allow the synthesis of
peptides or protein fragments, the American biochemist Robert Bruce Merrifield
developed a solid phase synthesis method for synthesizing peptides.
Dr. Merrifield devised a method
in which the last amino acid in the desired peptide chain was anchored
chemically to a fixed substrate. Each additional amino acid was added,
chemically linked by organic chemistry techniques. The system was washed free
of reaction products before adding the next amino acid. This synthetic method
proceeded from the COOH (carboxyl or C-terminal) end to the NH2 (amino or
N-terminal end). This direction is the opposite of natural protein synthesis.
In 1984, Merrifield received
the Nobel Prize in chemistry for his synthetic work. The basic Merrifield
method is still widely used today, however, mostly as itís Fmoc version.
Merrifield
method-based synthesis of a dipeptide
A functionalized resin is the
solid phase starting material for this synthesis method. The resin serves as an
anchor for the incoming amino acid.
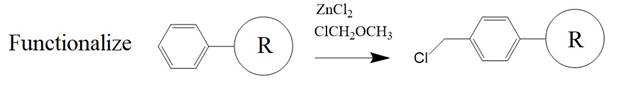
The basic solid-phase
synthesis starts with the desired carboxy-terminal amino acid (R1) blocked on
its amino terminus with an organic group, for example, the tert-butyloxycarbonyl- or BOC-group. The blocked amino acid is
conjugated or coupled to a chloromethyl group on the functionalized
resin.
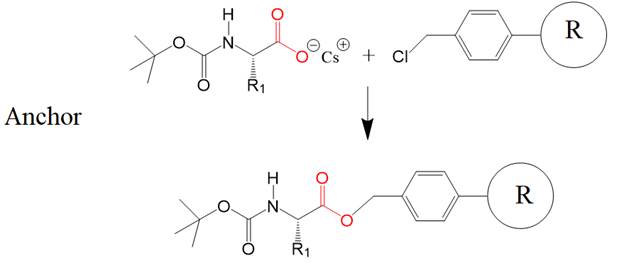
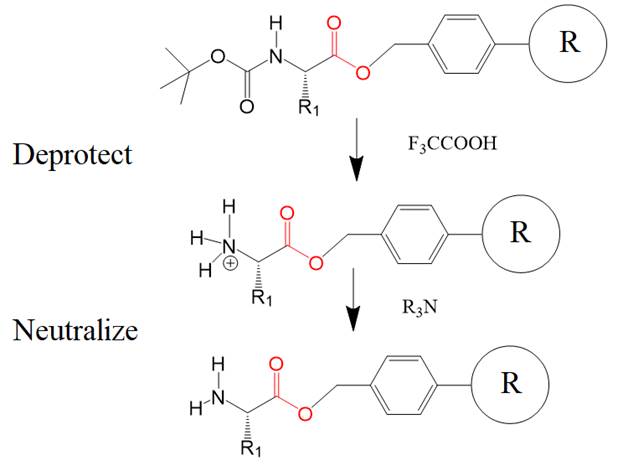
The next step removes the
blocking group to allow receiving the second amino acid (R2), also blocked on
its N-terminal end. Treatment with trifluoroacetic acid removes the blocking
group. The positively charged amino group is neutralized with a base to allow
efficient coupling of the next incoming blocked amino acid.
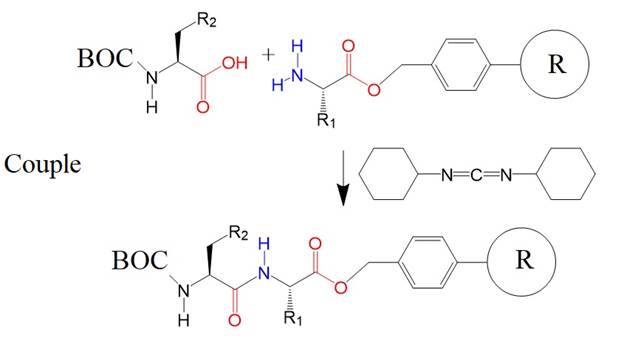
The second amino acid is
coupled to the resin-bound R1 residue with the help of dicyclohexylcarbodiimide.
The coupling and deprotection steps can be repeated over and over as desired.
Washing steps are included to achieve a purer product.
The final peptide is cleaved
of the resin using hydrofluoric acid (HF) and purified.
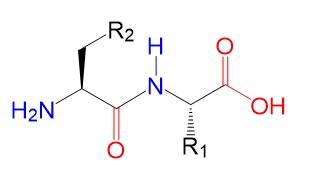
Final Dipeptide.
However, modern routine
automated peptide synthesis now heavily utilizes the
Fluorenyl-9-methoxycarbonyl (Fmoc) based solid-phase peptide synthesis method.
Modern Automated Solid
Phase Peptide Synthesis
Automated Solid Phase Peptide
Synthesis (SPPS) is now a very efficient and fast method for producing modified
and un-modified synthetic peptides. SPPS assembles peptides starting with
Fmoc-protected amino acids anchored by their c-terminal ends to an insoluble
polymer resin. The final assembled peptide contains the amino acid sequence
correlating to the successive assembly of the protected amino acid monomers. An
amino acid residue is added to the previous amino acid during each synthesis
cycle. In some cases, it is now also possible to synthesize peptides in length
up to 100 amino acid residues or longer. Synthesis proceeds from the C-terminal
end to the N-terminal end of the peptide. During Fmoc-based SPPS strategies, a
base labile Nα protecting group is used. All other protecting groups for
side-chain protection are acid-labile.
Reference
R. B. Merrifield (1963),
"Solid Phase Peptide Synthesis. I. The Synthesis of a
Tetrapeptide", Journal of the American Chemical Society, 85 (14):
2149Ė2154, doi:10.1021/ja00897a025